- Research article
- Open access
- Published:
Diagnostic behaviour of general practitioners when suspecting Lyme disease: a database study from 2010-2015
BMC Family Practice volume 19, Article number: 43 (2018)
Abstract
Background
Due to the raised public awareness of Lyme Borreliosis (LB), its increased incidence and the increased availability of serological tests, the demand for diagnostic testing on LB has increased. This may affect the diagnostic behaviour of general practitioners (GPs). Aim of our study was to describe GPs’ diagnostic behaviour when suspecting LB.
Methods
In this descriptive study from January 2010 to June 2015, we used the anonymized electronic medical records of 56,996 patients registered in 12 general practices in Amsterdam, The Netherlands. The target population was identified by means of an extensive search strategy, based on International Classification of Primary Care (ICPC-1) codes, free text and diagnostic test codes. All contacts related to LB were included in the analysis.
Results
2311 patients were included, accounting for 3861 LB contacts and 2619 LB episodes. The distribution of LB contacts showed annual peaks during spring and summer. Serological testing was performed in 36.4% of LB episodes and was mostly requested in patients presenting with general symptoms (71.4%). Unnecessary testing often occurred and only 5.9% of the tests turned out to be positive by immunoblot. From January 2010 to June 2015, no significant differences were found in the number of requested serological tests. The level of serological testing during LB episodes differed significantly between the general practices (19.2% to 75.8%).
Conclusions
Contrary to clinical guidelines, GPs regularly requested serology even when there was a low suspicion of LB. The development of an easy-to-use diagnostic algorithm may decrease overuse of diagnostic tests and thereby reduce overtreatment of LB.
Background
Lyme Borreliosis (LB) is the most common tick-borne disease in the world [1,2,3,4] caused by Borrelia burgdorferi sensu lato-spirochetes, of which Borrelia afzellii and Borrelia garinii are most prevalent in Europe [1, 5]. Over the past decades, the incidence of LB in Europe has increased to approximately 65,500 patients per year [6,7,8,9]. Raised public awareness of LB, its various clinical manifestations, its treatable character, the fear of disease among patients and the increased availability of serological tests have driven a rising demand for diagnostic testing [8, 10, 11]. Current European guidelines, although not tailored to primary care, clearly state that serological tests must only be done when there is a high suspicion of LB. This high a priori probability is mainly based on LB-specific symptoms (like symptoms suggesting Lyme arthritis or neuroborreliosis) [12, 13]. Requesting non-indicated tests may lead to false-positive results, since - depending on the assay - 3-9% of healthy controls is seropositive for Borrelia antibodies [14]. This is because serological tests do not differentiate between an active LB and an (asymptomatic) infection from the past [11]. Other reasons for a false-positive test result are infections with other related pathogens (e.g. syphilis), autoimmune disorders, or cross-reactivity between spirochetes. [15] In general, according to Bayes’ theorem, the usefulness of a serological test for LB depends on the pre-test probability and the subsequent predictive values in the setting where the test is being used [2, 16, 17]. However, even in a tertiary, multidisciplinary setting it is challenging to either rule out or demonstrate an association with Borrelia burgdorferi sensu lato. [18] The seropositivity rates for LB vary throughout Europe, from 3.4% in the general population in Italy to 15.2% in France [14, 19, 20]. When testing high-risk populations, these seropositive rates will be even higher. In Poland, healthy forestry workers reach a seropositivity rate of 45% [19, 21]. Because positive test results are often interpreted as Borrelia being the causal agent of the symptoms, overtreatment is common in cases of false-positive test results. Previous studies in general practices have only focused on tick bites and erythema migrans (EM). In Belgium 50% of the patients presenting with a typical EM got serologically tested by the GP [9], contrary to national/international guidelines (i.e. patients presenting with EM should always receive antibiotic treatment, so serological testing has no additional value; moreover, a false negative test result may occur, which can be misleading [12, 13].
The aim of the present study was to describe GPs’ diagnostic behaviour with respect to LB. Our study is the first to describe seasonal trends in LB contacts, to link symptoms to serology requests, and to investigate if GPs’ diagnostic behaviour on LB has changed over the years 2010-2015.
Methods
An observational study was carried out with anonymized data extracted from the database of the Academic Network of General Practice of VU University Medical Center (ANH VUmc), Amsterdam. The database contains pseudonimized primary health care data. We used the anonymized data of 56,996 patients registered in 12 general practices (with more than 60 GPs) in Amsterdam, from January 2010 to June 2015. In the Netherlands, almost all non-institutionalised citizens are registered to a general practice and the GP acts as gatekeeper. We developed an extensive search and selection strategy, based on the International Classification of Primary Care (ICPC-1) codes, free text and diagnostic test codes, to identify contacts related to LB (see Fig. 1 for the flow chart, Appendix 1 for the search strategy, and Appendix 2 for the origin of the identified patients, divided by search strategy). Annotations of the GPs from all identified contacts (consultations, telephone, home visits, e-mails) were reviewed by one of the investigators (EB). Contacts not related to LB or tick bites were excluded (e.g. other insect bites). A random selection of 5% of the identified contacts was reviewed by a second, independent reviewer to check the reliability of the data extraction. Disagreements were resolved during a consensus meeting between investigator and independent reviewer.
The following data were extracted from the selected medical records (analytical sample): basic characteristics of the patients (i.e. sex, age at the time of the first LB contact), characteristics of the LB contact and episode (i.e. date of contact, type of contact, number of contacts), ICPC-1 code, symptoms registered, findings during physical examination, diagnosis and management (i.e. Lyme serology or referral). Contacts belonging to the same episode were merged for the analysis of presenting symptoms and diagnostic testing. In the analysis, a distinction was made between individual contacts and episodes that include all contacts concerning the same complaint. Also, a distinction was made between a definite tick bite and a possible tick bite; the latter was called ‘insect sting’ in the analysis.
The data contained different Lyme serology tests from different laboratories and it was not possible to identify the exact assay that was used for each patient. Based on the quantitative value and the cut-off level we could infer that the majority of the samples were screened with the Immunetics C6 ELISA kit (Immunetics Inc., Boston, USA). We used the available laboratory interpretation of the screening test. This included the requirement that all equivocal or positive screening test results had to be confirmed by an immunoblot. Laboratories classified immunoblot outcomes as ‘negative’, ‘inconclusive’ or ‘positive’.
Descriptive analysis was performed using SPSS 23.0. Chi-square tests for trend (linear-by-linear) were used for comparing differences between years regarding contacts, episodes and serological tests.
The ANH database is run according to Dutch privacy legislation and contains pseudonymized general practice care data from all patients of the participating general practices, excluding those patients who object to this. Observational studies based on anonymized data from the ANH VUmc database are exempted from informed consent of patients.
Results
Figure 1 provides a flowchart of the study. Twelve general practices were included (N = 56,996 patients), resulting in 2311 patients who visited the GP because of complaints or concerns related to LB in the period January 2010 to June 2015 (see Appendix 3 for practice characteristics). The reliability of the data extraction was acceptable: from a random selection of 5% of identified LB contacts, 6 out of 350 contacts (1.7%) had been classified incorrectly.
The basic characteristics of patients and their LB contacts are shown in Table 1.
The overall incidence of episodes related to LB was 8.8 per 1000 person years within the dataset of 56,996 patients. The incidence of LB episodes across the years 2010-2014 did not show a significant change (p = 0.932). Since data for 2015 were only partially available, 2015 was not included in this analysis. Each year, LB related contacts showed a peak during spring and summer (May until September; Fig. 2). The majority of patients (78.2%) contacted their GP only once within an LB episode. For LB episodes with 2 or more contacts, the mean interval between first and last contact was 45 days. Most patients (88%) experienced only one LB episode over the period January 2010 to June 2015.
Table 2 provides an overview of reasons for encounter during episodes related to LB (N = 2619 episodes). Also, the table illustrates per reason for encounter how often serological testing was ordered and how often this resulted in a positive immunoblot test result.
Symptoms most frequently registered by GPs in LB episodes were recent insect sting, defined as ‘recent’ when stung within three months prior to contact (N = 1162, 44.4% of episodes), skin abnormalities (N = 1080, 41.2%) and general symptoms like fatigue and malaise (N = 966, 36.9%; Table 2).
Lyme serology was requested during 953 out of 2619 episodes (36.4%). GPs mostly requested serology for episodes in which patients presented with general symptoms (N = 690, 71.4%). The percentage of positive test results was highest for the episodes in which patients presented with skin abnormalities (14.5%) and for episodes in which patients requested for a test themselves (13.4%; Table 2). GPs requested serology for 18.4% (N = 25 out of 136) of the episodes in which patients presented with typical EM, for 10.1% (N = 33 out of 327) of the episodes in which patients presented with a locally irritated tick bite, and for 13.2% (N = 121 out of 914) of the episodes in which patients presented with an asymptomatic tick bite.
From January 2010 to June 2015, the number of requested serological tests did not change significantly (p = 0.190). Of the 953 serological tests, 19.3% was screened positively. Only 5.9% of these were confirmed by the immunoblot. Referral to a specialist occurred in 7.6% of all LB episodes, mostly to neurologists (24.2%) or internists (21.7%; Table 3).
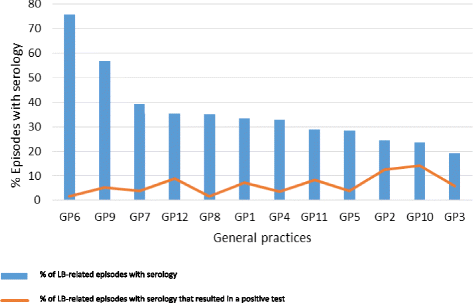
The general practices showed considerable differences with respect to serological testing during LB episodes, ranging from 19.2% to 75.8% of the LB episodes. There was a tendency for practices with lower serological testing rates to have higher positive tests rates (Fig. 3).
Discussion
Summary of main findings
This study was performed to describe GPs’ diagnostic behaviour with respect to LB. As expected, LB contacts showed annual peaks during spring and summer. Of all episodes, 36.4% were followed by a serological test for LB. Tests were mainly performed for episodes in which patients presented with nonspecific complaints like fatigue and headache. Contrary to clinical guidelines, a serological test was performed in 18.4% of the episodes with a typical EM, 10.1% of the episodes with a locally irritated tick bite, and 13.2% of the episodes with an asymptomatic tick bite. Overall, only 5.9% of the serological tests turned out to be positive which is as high as the seropositivity rate of LB in the general population [14]. Also, considerable differences between the 12 general practices were found regarding the rates of serological requests. The number of LB contacts and requested serological tests did not change over the period January 2010 to June 2015.
Strengths and weaknesses
This study is the first to explore seasonal trends in LB contacts, to link symptoms with serology requests, and to investigate time trends in GPs diagnostic behaviour on LB over a period of four-and-a-half years. A strength of our study is the use of an extensive, combined search strategy (see Appendix 1). Such a search strategy is crucial, as single search strategies – based on either free text, ICPC-1 code, or diagnostic testing code – all would miss a significant proportion of the target population (see Appendix 2). For example, if one would not use the free text search, 610 patients (26.4% of all 2311 identified patients) and 1023 contacts (27.8% of all 3681 identified contacts) would have been missed. This would have led to an overestimation of the level of serological testing during LB related episodes. Some potential limitations need to be considered. Firstly, the quality of the data depends on the completeness and accuracy of registration by the GP and our selection and data extraction strategy. Relevant contacts may have been missed, which would then have resulted in an underestimation of the actual contact frequency for LB. However, we expect this to be unlikely, because we did not only rely on ICPC-1 codes but also included the free text annotations - which were mined on relevant search terms - and diagnostic testing codes. In addition, GPs affiliated to the ANH VUmc database receive regular training in EMR coding and registration. Secondly, episodes in which both patient and GP did not suspect nor mention LB were not included. Therefore, we were not able to measure underdiagnosis, i.e. not requesting a test although indicated. Finally, we studied data from general practices in an urban area, which may limit the generalizability to rural areas, or other (European) areas, given the reported increasing gradient from west to east (highest in central-eastern Europe), and decreasing gradient from south to north in Scandinavia and from north to south in Italy, Spain and Greece [20].
Comparison with existing literature
The seropositive prevalence of LB in the general Dutch population is 5-10% [11, 12, 14, 22]. Given the low percentage of seropositive patients (5.9%) in our study population, it is likely that GPs regularly requested serology when there only was a low suspicion on LB [23]. This is substantiated by the finding that GPs mostly requested serology for episodes in which patients presented with general/nonspecific symptoms. According to the current guidelines, EM and asymptomatic tick bites should not be followed by a serological test on LB [12, 13]. Nevertheless, the GPs in our sample performed serological testing in one fifth of all patients with EM. This may seem substantial, but other international studies present much higher percentages, i.e. 50% and 68% in Belgium and France, respectively [9, 24]. Also, 121 out of 914 (13.2%) asymptomatic tick bites were serologically tested. In Belgium, 17.5% of the asymptomatic tick bites got serologically tested [9].
In our study, serology was mostly requested for episodes in which patients presented with nonspecific symptoms. Previous studies showed that unexplained symptoms, other than LB, more frequently lead to laboratory testing by the GP [25, 26]. Reasons for ordering tests for patients with unexplained symptoms may be a limited tolerance to diagnostic uncertainty or time pressure [25]. Once a laboratory test has been requested, the threshold for more testing is low and many GPs are unaware of the consequences (like false positive findings) [25, 26]. For example, an Australian study showed that 64.2% of patients presenting with fatigue to their GP received laboratory testing. In only 4% of the patients the tests led to a significant clinical diagnosis [26]. Improving GPs’ knowledge on the subject, combined with an easy-to-use diagnostic algorithm may increase confidence and reduce the overuse of diagnostic tests.
The large differences in serological testing between general practices suggest that - despite multidisciplinary guidelines - there is no consensus how to apply these guidelines in daily general practice and when to perform serological testing on LB.
Implications for clinicians and research
Hopefully, our study will initiate the development of an easy-to-use diagnostic algorithm for LB that is (also) applicable in daily general practice. Although the current scientific evidence for such an algorithm is limited, some promising potential predictors have been reported, like tick engorgement and patient-estimated duration of tick attachment. [10, 27] Future research may focus on the needs and expectations of patients and GPs during LB consultations, for example by a focus group study or semi-structured interviews. This may reveal clues to arrive at optimal (diagnostic) healthcare decisions with respect to LB.
Conclusions
This descriptive GP database study, covering the period January 2010 to June 2015, was the first to investigate seasonal trends in LB contacts, to link symptoms to serology requests, and to investigate GPs’ diagnostic behaviour over time. The distribution of LB contacts showed annual peaks during spring and summer. Episodes in which patients presented with general symptoms, like fatigue, were most frequently followed by serological testing. Serological testing was performed in 36.4% of LB episodes; only 5.9% of these tests turned out to be positive. The high number of LB consultations with nonspecific complaints together with the low frequency of positive serological tests, and the considerable inter-practice differences indicate a high number of inappropriate tests and underscores the need for an easy-to-use diagnostic algorithm that is (also) applicable in daily general practice.
Abbreviations
- ANH VUmc:
-
Academic Network of General Practice of VU University Medical Center
- CI:
-
Confidence Interval
- EM:
-
Erythema migrans
- GP:
-
General Practitioner
- ICPC-1:
-
International Classification of Primary Care
- LB:
-
Lyme Borreliosis
References
Sykes RA, Makiello P. An estimate of Lyme borreliosis incidence in Western Europe. J Public Health (Oxf). 2017;39(1):74–81.
Stanek G, Fingerle V, Hunfeld KP, Jaulhac B, Kaiser R, Krause A, Kristoferitsch W, O'Connell S, Ornstein K, Strle F, et al. Lyme borreliosis: clinical case definitions for diagnosis and management in Europe. Clin Microbiol Infect. 2011;17(1):69–79.
Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet. 2012;379(9814):461–73.
Steere AC, Strle F, Wormser GP, Hu LT, Branda JA, Hovius JW, Li X, Mead PS. Lyme borreliosis. Nat Rev Dis Primers. 2016;2:16090.
Rudenko N, Golovchenko M, Grubhoffer L, Oliver JH Jr. Updates on Borrelia burgdorferi sensu lato complex with respect to public health. Ticks Tick Borne Dis. 2011;2(3):123–8.
Brouwer ML, Rietmeijer-Mentink M, Sprong H, Van der Wouden JC, Bindels PJE. Tick bites: a dilemma in general practice. Huisarts Wet. 2013;56(7):5.
Hofhuis A, Harms M, Bennema S, van den Wijngaard CC, van Pelt W. Physician reported incidence of early and late Lyme borreliosis. Parasit Vectors. 2015;8:161.
Rizzoli A, Hauffe H, Carpi G, Vourc HG, Neteler M, Rosa R. Lyme borreliosis in Europe. Euro Surveill. 2011;16(27)
Vanthomme K, Bossuyt N, Boffin N, Van Casteren V. Incidence and management of presumption of Lyme borreliosis in Belgium: recent data from the sentinel network of general practitioners. Eur J Clin Microbiol Infect Dis. 2012;31(9):2385–90.
Ang W, Wolfs TFW. Diagnosis of Lyme borreliosis. Huisarts Wet. 2015;58(5):5.
Coumou J, Hovius JW, van Dam AP. Borrelia burgdorferi sensu lato serology in the Netherlands: guidelines versus daily practice. Eur J Clin Microbiol Infect Dis. 2014;33(10):1803–8.
Dutch Guideline Lyme Disease, July 2013. http://www.tekenbeetziekten.nl/wp-content/uploads/2014/08/CBO-richtlijn-Lymeziekte-versie-2013.pdf. Accessed on October 5th, 2017.
Belgian Guideline Lyme Borreliosis (Infection with Borrelia), 2016. Belgian Antibiotic Policy Coordination Committee (BAPCOC). http://overlegorganen.gezondheid.belgie.be/sites/default/files/documents/gids_lyme_borreliose_nl_march2017.pdf. Accessed on October 5th 2017.
Ang CW, Brandenburg AH, van Burgel ND, Bijlmer HA, Herremans T, Stelma F, Lunel FV, van Dam AP, Dutch Working Group on Diagnosis of Lyme B. A Dutch nationwide evaluation of serological assays for detection of Borrelia antibodies in clinically well-defined patients. Diagn Microbiol Infect Dis. 2015;83(3):222–8.
Lindsay LR, Bernat K, Dibernardo A. Laboratory diagnosis of Lyme disease. CCDR. 2014;40(11):9.
Leeflang MM, Ang CW, Berkhout J, Bijlmer HA, Van Bortel W, Brandenburg AH, Van Burgel ND, Van Dam AP, Dessau RB, Fingerle V, et al. The diagnostic accuracy of serological tests for Lyme borreliosis in Europe: a systematic review and meta-analysis. BMC Infect Dis. 2016;16:140.
Tugwell P, Dennis DT, Weinstein A, Wells G, Shea B, Nichol G, Hayward R, Lightfoot R, Baker P, Steere AC. Laboratory evaluation in the diagnosis of Lyme disease. Ann Intern Med. 1997;127(12):1109–23.
Coumou J, Herkes EA, Brouwer MC, van de Beek D, Tas SW, Casteelen G, van Vugt M, Starink MV, de Vries HJ, de Wever B, et al. Ticking the right boxes: classification of patients suspected of Lyme borreliosis at an academic referral center in the Netherlands. Clin Microbiol Infect. 2015;21(4):368. e311-320
Zakutna L, Dorko E, Mattova E, Rimarova K. Sero-epidemiological study of Lyme disease among high-risk population groups in eastern Slovakia. Ann Agric Environ Med. 2015;22(4):632–6.
Hubalek Z. Epidemiology of Lyme borreliosis. Curr Probl Dermatol. 2009;37:31–50.
Chmielewska-Badora J, Moniuszko A, Zukiewicz-Sobczak W, Zwolinski J, Piatek J, Pancewicz S. Serological survey in persons occupationally exposed to tick-borne pathogens in cases of co-infections with Borrelia burgdorferi, Anaplasma phagocytophilum, Bartonella spp. and Babesia microti. Ann Agric Environ Med. 2012;19(2):271–4.
Coumou J, van der Poll T, Speelman P, Hovius JW. Tired of Lyme borreliosis. Lyme borreliosis in the Netherlands. Neth J Med. 2011;69(3):101–11.
Lakos A, Igari Z, Solymosi N. Recent lesson from a clinical and seroepidemiological survey: low positive predictive value of Borrelia burgdorferi antibody testing in a high risk population. Adv Med Sci. 2012;57(2):356–63.
Letrilliart L, Ragon B, Hanslik T, Flahault A. Lyme disease in France: a primary care-based prospective study. Epidemiol Infect. 2005;133(5):935–42.
van der Weijden T, van Bokhoven MA, Dinant GJ, van Hasselt CM, Grol RP. Understanding laboratory testing in diagnostic uncertainty: a qualitative study in general practice. Br J Gen Pract. 2002;52(485):974–80.
Wilson J, Morgan S, Magin PJ, van Driel ML. Fatigue--a rational approach to investigation. Aust Fam Physician. 2014;43(7):457–61.
Hofhuis A, van de Kassteele J, Sprong H, van den Wijngaard CC, Harms MG, Fonville M, Docters van Leeuwen A, Simoes M, van Pelt W. Predicting the risk of Lyme borreliosis after a tick bite, using a structural equation model. PLoS One. 2017;12(7):e0181807.
Acknowledgements
Special thanks to all GPs who participate in the Academic Network of General Practice of VU University medical center (ANH VUmc) for extracting the data.
Funding
This study was not funded.
Availability of data and materials
The data that support the findings of this study are available from the ANH VUmc database but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of ANH VUmc database.
Author information
Authors and Affiliations
Contributions
EB, JCvdW and ORM were involved in the design of the study. EB performed the statistical analysis. EB wrote the original draft. All authors (EB, CWA, JHKJ, PS, JCvdW and ORM) made substantial contributions to interpretation of data, critically revised the draft for important intellectual content, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ANH database is run according to Dutch privacy legislation and contains pseudonymized general practice care data from all patients of the participating general practices, excluding those patients who object to this. Observational studies based on anonymized data from the ANH VUmc database are exempted from informed consent of patients. Permissions were sought and granted to access the ANH VUmc database.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1
Appendix 2
Appendix 3
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Botman, E., Ang, C.W., Joosten, J.H.K. et al. Diagnostic behaviour of general practitioners when suspecting Lyme disease: a database study from 2010-2015. BMC Fam Pract 19, 43 (2018). https://doi.org/10.1186/s12875-018-0729-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12875-018-0729-2